Abstract
Introduction: Persistently elevated fetal hemoglobin (HbF) can ameliorate transfusion-dependent beta thalassemia (TDT) (Musallam et al ., 2012). BCL11A is a repressor of γ-globin expression and HbF production in adult erythrocytes. Down-regulation of BCL11A is a therapeutic strategy for induction of HbF in beta hemoglobinopathies. In pre-clinical studies with human hematopoietic stem cells (HSCs), zinc finger nuclease (ZFN)-mediated disruption of the GATA-binding region of the intronic erythroid-specific BCL11A enhancer (BCL11A ESE) decreased BCL11A expression and increased HbF production in erythroid cells without disrupting multi-lineage hematopoiesis. ST-400 is an investigational cell therapy product comprised of autologous CD34+ cells that have undergone high-precision, ZFN-mediated ex vivo editing at the BCL11A gene ESE target. The aim of this study is to induce HbF expression in edited erythroid cells. We hypothesized that infusion of ST-400 after myeloablative conditioning would have a favorable benefit risk profile in TDT.
Methods: The Thales trial (NCT03432364) is a Phase 1/2 study of the safety, tolerability and efficacy of ST-400 in adult subjects with TDT, defined as receiving ≥8 annual red blood cell transfusion episodes for at least 2 consecutive years before enrollment. Leukapheresis was performed following mobilization with G-CSF and plerixafor. Autologous collections were enriched for CD34+ cells and then transfected with mRNA encoding ZFNs with binding sites flanking the GATA-binding region of BCL11A ESE. The ST-400 product was infused following myeloablative busulfan conditioning. Six subjects were to be monitored for safety and efficacy for 3 years post-infusion.
Results: Five subjects (age 18 to 36 years old, average 28 years) have completed ST-400 manufacturing and have been infused. No additional patients will be infused. Subjects received an average of 7.3 x 10 6 CD 34+ cells/kg (high low 4.5-11.4). The first subject experienced a serious adverse event (SAE) of hypersensitivity soon after the initiation of ST-400 infusion that was considered by the investigator to be related to the ST-400 infusion, most likely to dimethylsulfoxide (DMSO). The event of hypersensitivity was successfully medically managed and the patient received the full study dose after brief interruptions. One other treatment emergent SAE (opioid withdrawal) occurred, assessed by the investigator as unrelated to ST-400. As of 03 May 2021, the majority of the AEs reported following treatment with ST-400 are consistent with myeloablation. No clonal expansion has been observed from monitoring on-target indel clones longitudinally.
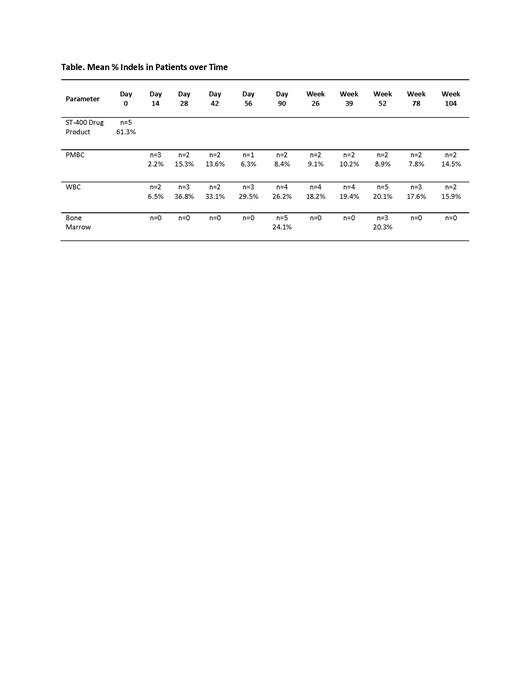
All five subjects had prompt hematopoietic reconstitution (average ANC recovery day +18 [range 14-24 days]; platelet recovery day +29 [range 19-44 days]), with increasing HbF fraction that contributed to stable total hemoglobin. As of 03 May 2021, with follow-up ranging from 17.6 to 27.3 months, on-target DNA insertions-deletions (indels) at BCL11A ESE were present in peripheral blood mononuclear cells (PBMCs) of all five subjects.
Peak HbF levels achieved were 23.5 +- 11.4% (high low 35.9-6.9%) but were not sustained, and thus were not sufficient for transfusion independence. At the time of the latest visit (65-117 weeks), the HbF levels are 7.7 +- 4.1% (high low 13.5-2%). This decline is presumably due to low levels of long-term progenitors in the final drug product.
Conclusions: ST-400 is an ex vivo, ZFN-edited autologous HSC product candidate for increased erythroid HbF expression in patients with TDT. All five infused subjects had rapid hematopoietic reconstitution following myeloablative conditioning, on-target indels in PBMCs and elevated HbF levels following HSCGT. After 1 to 2 years, the average HbF levels had declined by 64% from peak to last visit, which necessitated resumption of PRBC transfusions at the same rate as prior to the ST-400 therapy. The data in the 5 patients infused indicate that the procedure was generally well tolerated and was associated with a significant albeit transient increase in HbF after reconstitution.
Walters: Ensoma, Inc.: Consultancy; Vertex pharmaceuticals: Consultancy; AllCells, Inc: Consultancy; BioLabs, Inc: Consultancy. Smith: Astellas Gene Therapies: Current Employment. Schiller: ASH foundation: Other: Chair-unpaid; Pfizer: Current equity holder in publicly-traded company, Research Funding; Sanofi: Honoraria, Research Funding, Speakers Bureau; Ambit: Research Funding; Biomed Valley Discoveries: Research Funding; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Kaiser Permanente: Consultancy; MedImmune: Research Funding; Mateon: Research Funding; Cyclacel: Research Funding; PrECOG: Research Funding; Novartis: Consultancy, Research Funding; Onconova: Research Funding; Eli Lilly: Research Funding; Samus: Research Funding; Takeda: Research Funding; Jazz: Consultancy, Honoraria, Research Funding, Speakers Bureau; Stemline Therapeutics, Inc.: Honoraria, Research Funding, Speakers Bureau; Pharma: Consultancy; Johnson & Johnson: Current equity holder in publicly-traded company; Sangamo: Research Funding; Sellas: Research Funding; Ono: Consultancy; Leukemia & Lymphoma Society: Research Funding; AstraZeneca: Consultancy; Ariad: Research Funding; Incyte: Consultancy; Regimmune: Research Funding; Ono-UK: Consultancy, Research Funding; Bio: Research Funding; Elevate: Research Funding; Amgen: Consultancy, Current equity holder in publicly-traded company, Honoraria, Research Funding, Speakers Bureau; Tolero: Research Funding; Agios: Consultancy, Research Funding, Speakers Bureau; Trovagene: Research Funding; Karyopharm: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Geron: Research Funding; Genentech-Roche: Research Funding; Gamida Cell Ltd.: Research Funding; FujiFilm: Research Funding; Forma: Research Funding; Delta-Fly: Research Funding; Deciphera: Research Funding; Daiichi-Sankyo: Research Funding; Constellation Pharmaceuticals: Research Funding; Celator: Research Funding; BMS/Celgene: Consultancy, Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; Astellas: Honoraria, Research Funding, Speakers Bureau; Arog: Research Funding; Actuate: Research Funding; Actinium Pharmaceuticals, Inc: Research Funding; Abbvie: Research Funding; Bluebird Bio: Research Funding; Boehringer-Ingleheim: Research Funding; Cellerant: Research Funding; CTI Biopharma: Research Funding; Janssen: Research Funding; Kura Oncology: Research Funding; Pharmacyclics: Honoraria, Speakers Bureau; Millennium: Research Funding; National Marrow Donor Program: Research Funding; NIH: Research Funding; Onyx: Research Funding; Pharmamar: Research Funding; UC Davis: Research Funding; UCSD: Research Funding; Evidera: Consultancy; NCI: Consultancy; Novartis: Speakers Bureau. Esrick: bluebird bio: Consultancy. Williams: Beam Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Scientific Advisory Board; Emerging Therapy Solutions: Membership on an entity's Board of Directors or advisory committees, Other: Chief Scientific Chair; Geneception: Membership on an entity's Board of Directors or advisory committees, Other: Scientific Advisory Board; BioMarin: Membership on an entity's Board of Directors or advisory committees, Other: Insertion Site Advisory Board; Alerion Biosciences: Other: Co-founder (now licensed to Avro Bio, potential for future milestones/royalties); Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee, Novartis ETB115E2201 (eltrombopag in aplastic anemia). Advisory fees donated to NAPAAC.; Orchard Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Membership on a safety advisory board (SAB): SAB position ended 05/20/2021. Co-founder , Patents & Royalties: Potential for future royalty/milestone income, X-SCID. Provided GMP vector for clinical trial, Research Funding; bluebird bio: Membership on an entity's Board of Directors or advisory committees, Other: Insertion Site Analysis Advisory Board, Patents & Royalties: BCH licensed certain IP relevant to hemoglobinopathies to bluebird bio. The current license includes the potential for future royalty/milestone income. Bluebird has indicated they will not pursue this as a clinical program and BCH is negotiating return of, Research Funding. Gogoleva: Sangamo Therapeutics: Current Employment, Current equity holder in publicly-traded company. Rouy: Sangamo Therapeutics: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Ended employment in the past 24 months. Cockroft: Sangamo Therapeutics: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Vercellotti: Mitobridge, an Astellas Company: Consultancy, Research Funding; CSL Behring: Research Funding.
busulfan: used for myeloablation prior to infusing the investigational autologous HSPC product (ST-400) plerixafor: used with G-CSF to enhance mobilization of autologous HSPC for collection via leukapheresis. Autologous HSPC then undergo ex vivo manufacturing to generate the investigational product (ST-400)
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal